The ERA4Health objectives are to:
- Support relevant medical research including clinical fields and intervention areas (prevention, diagnosis, treatment).
- Improve the utilisation of existing health technologies in clinical practice.
- Build capacity, in particular in conducting Investigator Initiated Clinical Studies at EU scale.
- Implement and advance the practice of responsible research and innovation (RRI) across the breadth of the programme.
ERA4Health will be implemented in 2 phases:
- Phase 1:Joint calls focused on nutrition and lifestyle-related diseases, cardiovascular diseases and nanomedicine are implemented. Four calls are expected to be launched during the first two years of the partnership). The first two, Cardinnov and HealthEquity were launched in 2022 and have been closed for submission. The results for these are to be expected in the fall of 2023. The next two calls are currently being worked on and are expected to be launched in 2024.
- Phase 2. Additional joint calls focused on multinational investigator initiated clinical studies for other priority areas are expected to be launched in accordance with the decision of the Health Programme Committee and the European Commission. Updates on this matter will follow as soon as possible.

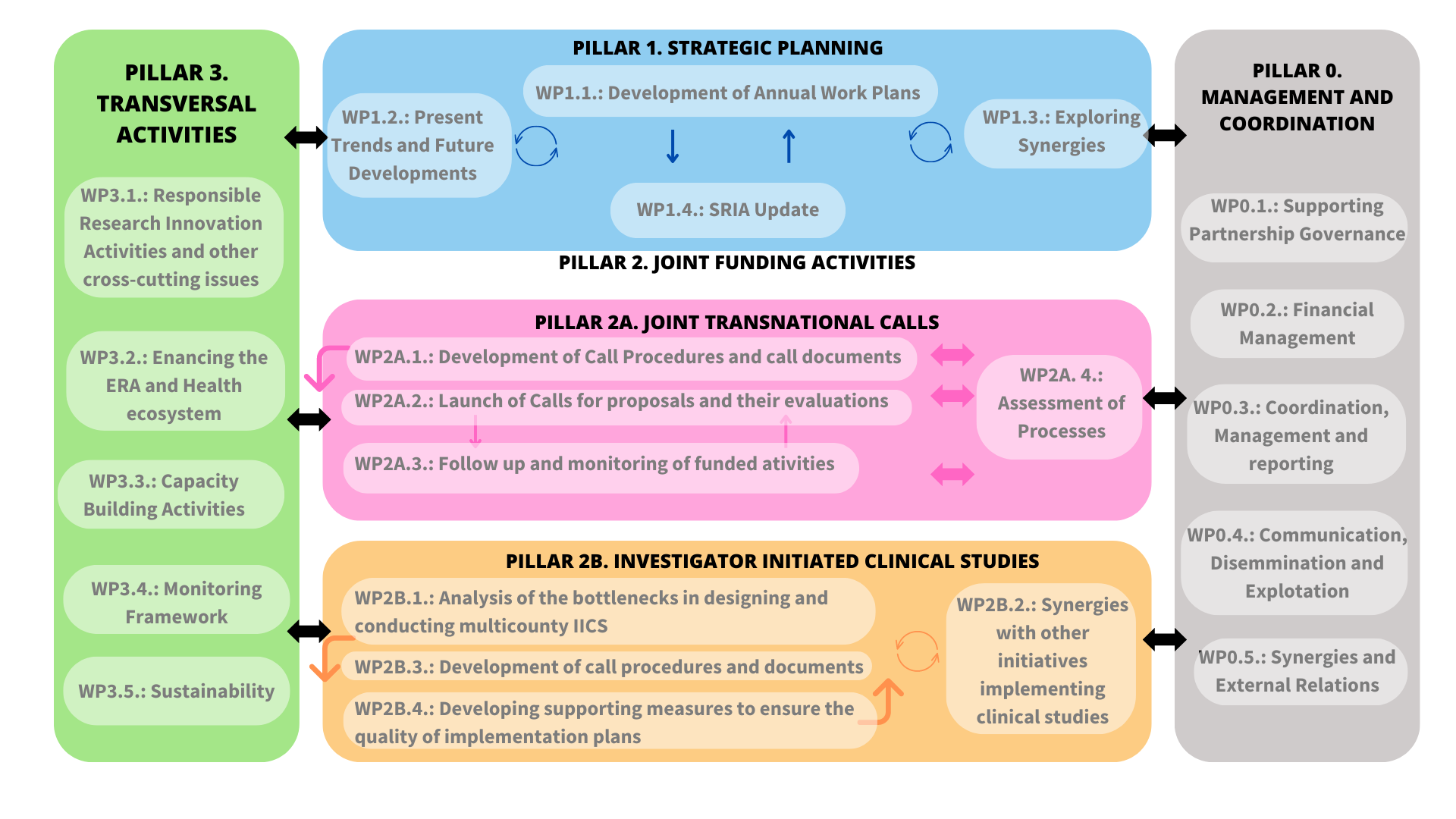
Work package structure
ERA4Health will pursue the previous objectives through the implementation of 22 interrelated work packages divided into 4 pillars:
- Pillar 0 for Management and Coordination
- Pillar 1 for Strategic Planning
- Pillar 2 for Joint Funding Activities
- Pillar 2A – Joint Transnational Calls
- Pillar 2B – Investigator Initiated Clinical Studies
- Pillar 3 for Transversal activities